Abstract
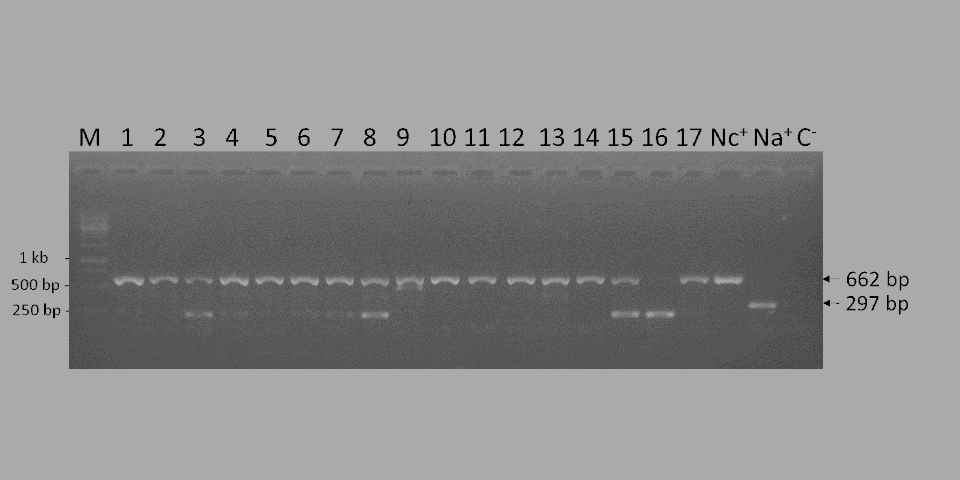
Nosema ceranae is a ubiquitous microsporidian pathogen infecting the midgut of honey bees. The infection causes bee nosemosis, a disease associated with malnutrition, dysentery, and lethargic behavior, and results in considerable economic losses in apiculture. The use of a rapid, sensitive, and inexpensive DNA-based molecular detection method assists in the surveillance and eventual control of this pathogen. To this end, a loop-mediated isothermal amplification (LAMP) assay targeting the singlecopy gene encoding the polar tube protein 3 (PTP3) has been developed. Genomic DNA of N. ceranae–infected forager bees sampled from distant geographic regions could be reliably amplified using the established LAMP assay. The N. ceranae-LAMP showed higher sensitivity than a classical reference PCR (98.6 vs 95.7%), when both approaches were applied to the detection of N. ceranae. LAMPdetected a ten-fold lower infection rate than the reference PCR (1 pg vs 10 pg genomic DNA, respectively). In addition, we show highly specific and sensitive detection of N. ceranae from spore preparations in a direct LAMP format. No cross-reactions with genomic DNA and/or spores from N. apis, often co-infecting A. mellifera, or from N. bombi, infecting bumble bees, were observed. This low-cost and time-saving molecular detection method can be easily applied in simple laboratory settings, facilitating a rapid detection of N. ceranae in honey bees in epidemiological studies, surveillance and control, as well as evaluation of therapeutic measures against nosemosis.